Going in to this lab I was prepared but nervous because it was either a pass or fail when being graded. I was under a lot of pressure ;). Somehow by studying the formula and looking at similar labs on YouTube I made it through the lab with flying colors. I had the smallest bag size and thought that would be the hardest but I'm glad I didn't have to stand at the sink the whole hour measuring the volume of the bag like others with the bigger bags had to do,
Tuesday, May 10, 2016
Avagadros law
Avagadros law tells us that for a gas at constant temperature and pressure, the volume is directly proportional to the number of moles of gas present. Equal volumes of gases at the same temperature and pressure have the same number of particles. V1/n1 = V2/n2
http://www.chemteam.info/GasLaw/Gas-Avogadro.html

http://www.chemteam.info/GasLaw/Gas-Avogadro.html

Boyle's Law
Boyle's law tells us that the relationship between pressure and volume is an inverse relationship. It deals with two of the four factors that determine the state of gas: pressure and volume. Temperatures are held constant. This is for ideal gases. As we increase in volume the pressure decreases. But as volume of a container decreases pressure will increase due to increase in collisions.
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html

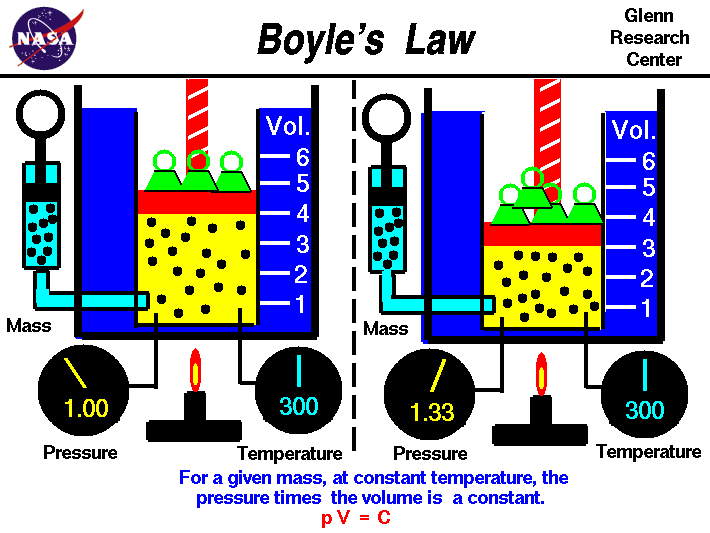
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html

Characteristics of gases
Gases expand spontaneously to fill their container. Gases are also highly compressible and form homogeneous mixtures. To measure the pressure of a gas a barometer is used. A simple barometer consists of a long glass tube, closed at one end and filled with mercury. There are a variety of ways to measure pressure and each have a different unit.
http://www.chem4kids.com/files/matter_gas.html
http://www.mikeblaber.org/oldwine/chm1045/notes/Gases/Intro/Gases01.htm
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch4/properties2.html
https://www.chem.purdue.edu/gchelp/liquids/character.html

http://www.chem4kids.com/files/matter_gas.html
http://www.mikeblaber.org/oldwine/chm1045/notes/Gases/Intro/Gases01.htm
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch4/properties2.html
https://www.chem.purdue.edu/gchelp/liquids/character.html
Energy in all forms
Thermodynamics is the study of energy transformations. In physics, energy is defined as the ability to do work(work=force/distance). Although we are unable to see or directly measure energy, we know that energy has the ability to make things happen. Two common forms of energy in chemistry are kenetic energy, the energy of motion, and potential energy, or stored energy.
Kinetic energy- energy of motion KE=1/2mv^2
Radiant energy- energy from the sun or solar energy
Thermal energy-energy associated with random motion of atoms and molecules
Chemical energy-energy stored within the structural units of chemical substances
Potential energy-energy stored or energy of position


Kinetic energy- energy of motion KE=1/2mv^2
Radiant energy- energy from the sun or solar energy
Thermal energy-energy associated with random motion of atoms and molecules
Chemical energy-energy stored within the structural units of chemical substances
Potential energy-energy stored or energy of position
Cooling and heating
Change of state is a physical change where intermolecular bonds are broken. Melting and boiling points are determined by vapor pressures of the solid and liquid states. At 0 degrees Celsius, ice and liquid water have the same vapor pressure. At 100 degrees Celsius, water vapor and atmospheric pressure are equal.
Super cooling is cooling of liquid below freezing point and it occurs when the particles lack the internal arrangement to solidify at freezing point.
Super heating is heating of a liquid is raised above its boiling point and it occurs when vapor bubbles fail to form and it may suddenly boil violently when vapor bubbles do form.
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/phase.html
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/watice.html
https://www.chem.wisc.edu/deptfiles/genchem/netorial/rottosen/tutorial/modules/intermolecular_forces/02imf/imf2.htm
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/changesOfPhase/changeOfState.html
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/changesOfPhase/changeOfState.html
http://www.shmoop.com/matter-properties/phase-change.html


Super cooling is cooling of liquid below freezing point and it occurs when the particles lack the internal arrangement to solidify at freezing point.
Super heating is heating of a liquid is raised above its boiling point and it occurs when vapor bubbles fail to form and it may suddenly boil violently when vapor bubbles do form.
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/phase.html
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/watice.html
https://www.chem.wisc.edu/deptfiles/genchem/netorial/rottosen/tutorial/modules/intermolecular_forces/02imf/imf2.htm
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/changesOfPhase/changeOfState.html
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/changesOfPhase/changeOfState.html
http://www.shmoop.com/matter-properties/phase-change.html
The U.S.S.
Once we found out we could use the remaining bio-diesel from other groups, we set sail in finding an idea that would make out putt-putt boat glide past the competition. we ended up making a model out of a soda can because it was light but also durable and was essentially water proof. We placed 4th and didn't get extra credit but we still felt we had a good team effort and planned everything perfectly.

The Making of our Biodiesel
My partner and I started making our bio-diesel and used the clean oil for the construction instead of the used Chick-fil-a oil. our mindset was that since it was cleaner the oil would burn hotter making our future boat run faster. Unfortunately, the solo cups we stored our biodiesel in disintegrated and all that was left was a mess that Mrs. Frankenberg had to clean. All hope is not yet lost. We know our biodiesel would have rocked based on our test burn.
was a mess that Mrs. Frankenberg had to clean. All hope is not yet lost. We know our biodiesel would have rocked based on our test burn.

Biodiesel Project
Today and all the next week we will be in the library computer lab working on our Bio diesel project. Unfortunately my partner wont be able to attend school so i will have to figure out a approach to our video and hope that we can finish in time. overall i'm excited about this project and hope to win the top prize.


http://biodiesel.org/
https://en.wikipedia.org/wiki/Biodiesel
http://www.afdc.energy.gov/fuels/biodiesel.html
https://www.fueleconomy.gov/feg/biodiesel.shtml
http://www.biodiesel.com/
http://www.biodieselmagazine.com/
http://biodiesel.org/
https://en.wikipedia.org/wiki/Biodiesel
http://www.afdc.energy.gov/fuels/biodiesel.html
https://www.fueleconomy.gov/feg/biodiesel.shtml
http://www.biodiesel.com/
http://www.biodieselmagazine.com/
Unit test
Although i studied for the test the whole week and understood the terminology and the mathematical side of it, i did poorly on the test next time i might look on quizlet for more practice. I have put links down below of what i would think are helpful.
https://quizlet.com/
http://www.visionlearning.com/en/library/Chemistry/1/Chemical-Bonding/55
https://en.wikipedia.org/wiki/Covalent_bond
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/Lewis_Theory_of_Bonding
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/lewis.html
https://www.khanacademy.org/science/chemistry/chemical-bonds/copy-of-dot-structures/v/drawing-dot-structures

https://quizlet.com/
http://www.visionlearning.com/en/library/Chemistry/1/Chemical-Bonding/55
https://en.wikipedia.org/wiki/Covalent_bond
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/Lewis_Theory_of_Bonding
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/lewis.html
https://www.khanacademy.org/science/chemistry/chemical-bonds/copy-of-dot-structures/v/drawing-dot-structures
Lewis Structures
Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Depending on the chemical make up of that bond, the Lewis structure may be different. Some Lewis structures appear flat such as the bond of water today we went to the library to draw Lewis structures on the dry erase tables.







All Shapes and Sizes
A chemical bond is a lasting attraction between atoms that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between atoms with opposite charges, or through the sharing of electrons as in the covalent bonds.
much of what we studied in class was the way the chemical bonds formed and the shapes they created below is a chart of the shapes bonds form
much of what we studied in class was the way the chemical bonds formed and the shapes they created below is a chart of the shapes bonds form
Friday, March 11, 2016
Energy Levels Change the Color
When you heat an atom, some of its electrons are "excited* to higher energy levels.
When an electron drops from one level to a lower energy level, it emits a quantum of energy.
3
The wavelength (colour) of the light depends on the difference in the two energy levels.
2
We can see only those transitions that correspond to a visible wavelength.
In a hydrogen atom, for example, we can see only the transitions from higher levels to n = 2 .
When an electron drops from one level to a lower energy level, it emits a quantum of energy.
3
The wavelength (colour) of the light depends on the difference in the two energy levels.
2
We can see only those transitions that correspond to a visible wavelength.
In a hydrogen atom, for example, we can see only the transitions from higher levels to n = 2 .
| As | Arsenic | Blue |
| B | Boron | Bright green |
| Ba | Barium | Pale/Yellow-green |
| Ca | Calcium | Orange-red |
| Cu (I) | Copper (I) | Blue |
| Cu (II) | Copper (II) non-halide | Green |
| Cu (II) | Copper (II) halide | Blue-green |
| Fe | Iron | Gold |
| In | Indium | Blue |
| K | Potassium | Light purple to red |
| Li | Lithium | Deep pink to dark red |
| Mg | Magnesium | Bright white |
| Mn (II) | Manganese (II) | Yellow-green |
| Mo | Molybdenum | Yellow-green |
| Na | Sodium | Bright yellow |
| P | Phosphorous | Pale blue-green |
| Pb | Lead | Blue |
| Rb | Rubidium | Red/Purple-red |
| Sb | Antimony | Pale green |
| Se | Selenium | Bright blue |
| Sr | Strontium | Crimson |
| Te | Tellurium | Pale green |
| Tl | Thallium | Bright green |
| Zn | Zinc | Blue-green to pale green |
Absorbency
https://drive.google.com/open?
id=0B6biT3nsiazTaDRxTGx1MW9CdGM
This is our lab activity that we measured absorbency and and transmitted. This lab was long and extensive but overall was very informative. I enjoyed how simply the procedure was and doing stuff on excel.
It's Lit
https://drive.google.com/open?id=0B6biT3nsiazTNkI1UnpsZjBaLW8
https://drive.google.com/open?id= 0B6biT3nsiazTcDBvZnN4YkpIQlE
This is our identifying the mystery metal was probably the best lab we've done so far. It was so fascinating how different metals produce different colors when burned. The colors were pretty as well . I wish we had more labs like this.
Trend Setters
Density Trends:
The density of an element is the amount of mass it has per unit volume. Normally this is measured in g cm−3 and at room temperature. Values are shown relative to osmium, the element with the highest density.
Atomic Radius trends:
The non-bonded atomic radius of an atom is half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. Atoms are not well defined spheres so there are many ways of calculating atomic radius. Values are shown relative to francium, the element with the highest atomic radius.
Electronegativity trends:
The electronegativity of an atom is how strongly it attracts electrons towards itself. It depends on the atomic radius and the atomic number of the element. Electronegativity is most commonly measured on the Pauling scale. Values are shown relative to fluorine, the element with the highest electronegativity.
Melting Point Trends:
The melting point of an element is the temperature at which the solid–liquid phase change occurs. Values are shown relative to the sublimation point of carbon, the highest temperature at which any element remains solid.
Ionization Energy trends:
The first ionisation energy of an atom is the minimum energy required to remove an electron from a neutral atom in its ground state. Values are shown relative to helium, the element with the highest first ionisation energy.
The density of an element is the amount of mass it has per unit volume. Normally this is measured in g cm−3 and at room temperature. Values are shown relative to osmium, the element with the highest density.
Atomic Radius trends:
The non-bonded atomic radius of an atom is half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. Atoms are not well defined spheres so there are many ways of calculating atomic radius. Values are shown relative to francium, the element with the highest atomic radius.
Electronegativity trends:
The electronegativity of an atom is how strongly it attracts electrons towards itself. It depends on the atomic radius and the atomic number of the element. Electronegativity is most commonly measured on the Pauling scale. Values are shown relative to fluorine, the element with the highest electronegativity.
Melting Point Trends:
The melting point of an element is the temperature at which the solid–liquid phase change occurs. Values are shown relative to the sublimation point of carbon, the highest temperature at which any element remains solid.
Ionization Energy trends:
The first ionisation energy of an atom is the minimum energy required to remove an electron from a neutral atom in its ground state. Values are shown relative to helium, the element with the highest first ionisation energy.
Useful Links
Periodic Trends Video
A few helpful links on periodic trends and electron configuration to aid in studying. These videos are easy to understand and educational.
Summary of Trends
This is the summary of periodic trends.Any atom or group of atoms with a net charge is called an ion. A positively charged ion is a cation while a negatively charged ion is an anion. Now we are ready to discuss the periodic trends of atomic size, ionization energy, electron affinity, and electronegativity
Think Pink
This is an image of the titrated solution when too much base is added. There is a fine line from the time you add the perfect amount of solution to one drop extra that makes a bright pink color. In order to get the perfect amount it takes trial and error.
Acid
Must See Video of Titration Lab
Above is a link of our titration lab. You can see the acid solution change colors as the base is added.it took careful concentration to avoid getting the solution too pink so the slower the better.
Above is a link of our titration lab. You can see the acid solution change colors as the base is added.it took careful concentration to avoid getting the solution too pink so the slower the better.
Titration
The word titration rhymes with tight nation. The medical use of this word is a little different, but in chemistry titration refers to a commonly used method of finding the concentration of an unknown liquid, by comparing it with a known liquid. An acid-base titration is good to consider when learning the method, but there are more uses for the technique. The measure of oxalate ion using potassium permanganate in a warm acid environment is a good example of a redox titration. The Mohr titration is a determination of chloride concentration using known silver nitrate solution and sodium dichromate an indicator.
Acid or Base Buffers
A buffer is a solution that resists changes in pH. A buffer is made with a weak acid and a soluble salt containing the conjugate base of the weak acid or a weak base and a soluble salt containing the conjugate acid of the weak base. Some examples of buffer material pairs are:
acetic acid and sodium acetate, H(C2H3O2) and Na(C2H3O2)
Weak Acid and Bases
Weak acids and bases do not ionize very much, so the [H+] or [(OH)-] must be calculated by the equilibrium expression.
Acid/Bases
An acid is a material that can release a proton or hydrogen ion. Hydrogen chloride in water solution ionizes and becomes hydrogen ions and chloride ions. a base, or alkali, is a material that can donate a hydroxide ion. Sodium hydroxide in water solution becomes sodium ions and hydroxide ions. Every ion dissociation that involves a hydrogen or hydroxide ion could be considered an acid- base reaction. Acids are electron pair acceptors and bases are electron pair donors. Each ionizable pair has a proton donor and a proton acceptor. Acids are paired with bases. One can accept a proton and the other can donate a proton. Each acid has a proton available and another part, called the conjugate base. When the acid ionizes, the hydrogen ion is the acid and the rest of the original acid is the conjugate base.
Solutions
http://study.com/academy/lesson/aqueous-solution-definition-reaction-example.html#lesson
Above is a very helpful video that explains examples of aqueous solutions. The definition is provided and it really dumbs down the lesson plan to where its easily interpreted by anyone.
Tuesday, January 26, 2016
Useful Links
Here are some helpful links that may help on this unit!
USC.edu - solubility calculator provides Ksp and solubility rules for lots of compound
uwaterlood.ca - how & why things dissolve including discussion of enthalpy (energy) involved
chemteam.info - units of concentration & colligative properties - tutorials and problem sets
USC.edu - comparing Lewis structures, VSEPR results, and visual models (requires chime)
avogadro.co.uk - explaining the crystal lattice with models
ausetute.com.au - how to write Lewis symbols for atoms & molecules
ccbcmd.edu - defining & assigning formal charge
USC.edu - solubility calculator provides Ksp and solubility rules for lots of compound
uwaterlood.ca - how & why things dissolve including discussion of enthalpy (energy) involved
chemteam.info - units of concentration & colligative properties - tutorials and problem sets
USC.edu - comparing Lewis structures, VSEPR results, and visual models (requires chime)
avogadro.co.uk - explaining the crystal lattice with models
ausetute.com.au - how to write Lewis symbols for atoms & molecules
ccbcmd.edu - defining & assigning formal charge
Murder Mystery Lab Day 2
We started the lab today and followed the instructions clearly and organized. As we worked through the lab we took necessary precautions and mixed the mystery solution with Na2CO3. We had to wait for the mixture to fully filter since a precipitate was formed. We concluded that Mr. Green was the murderer.
Murder Mystery Lab
We started preparing the murder mystery lab by creating a table of all the suspects and what substance they possessed. We thought by doing this we would have an easier time identifying the murderer once the lab was completed. I hope that this organization would lead to success.

Subscribe to:
Comments (Atom)




