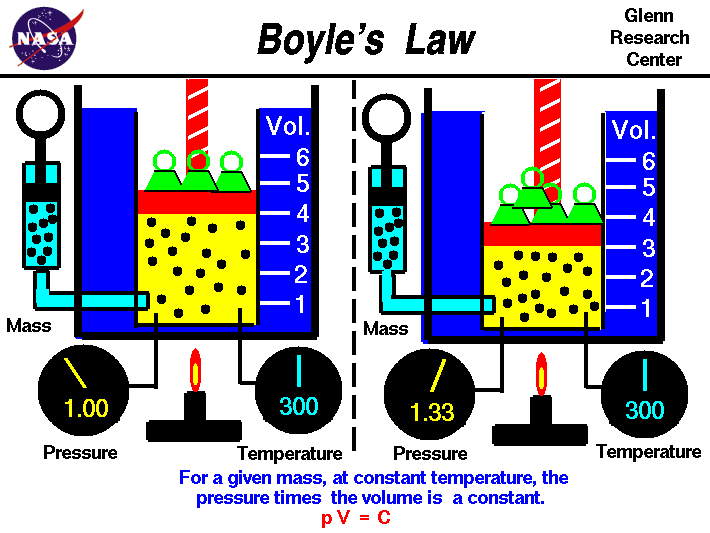
Boyle's law tells us that the relationship between pressure and volume is an inverse relationship. It deals with two of the four factors that determine the state of gas: pressure and volume. Temperatures are held constant. This is for ideal gases. As we increase in volume the pressure decreases. But as volume of a container decreases pressure will increase due to increase in collisions.
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html


No comments:
Post a Comment