Going in to this lab I was prepared but nervous because it was either a pass or fail when being graded. I was under a lot of pressure ;). Somehow by studying the formula and looking at similar labs on YouTube I made it through the lab with flying colors. I had the smallest bag size and thought that would be the hardest but I'm glad I didn't have to stand at the sink the whole hour measuring the volume of the bag like others with the bigger bags had to do,
Tuesday, May 10, 2016
Avagadros law
Avagadros law tells us that for a gas at constant temperature and pressure, the volume is directly proportional to the number of moles of gas present. Equal volumes of gases at the same temperature and pressure have the same number of particles. V1/n1 = V2/n2
http://www.chemteam.info/GasLaw/Gas-Avogadro.html

http://www.chemteam.info/GasLaw/Gas-Avogadro.html

Boyle's Law
Boyle's law tells us that the relationship between pressure and volume is an inverse relationship. It deals with two of the four factors that determine the state of gas: pressure and volume. Temperatures are held constant. This is for ideal gases. As we increase in volume the pressure decreases. But as volume of a container decreases pressure will increase due to increase in collisions.
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html

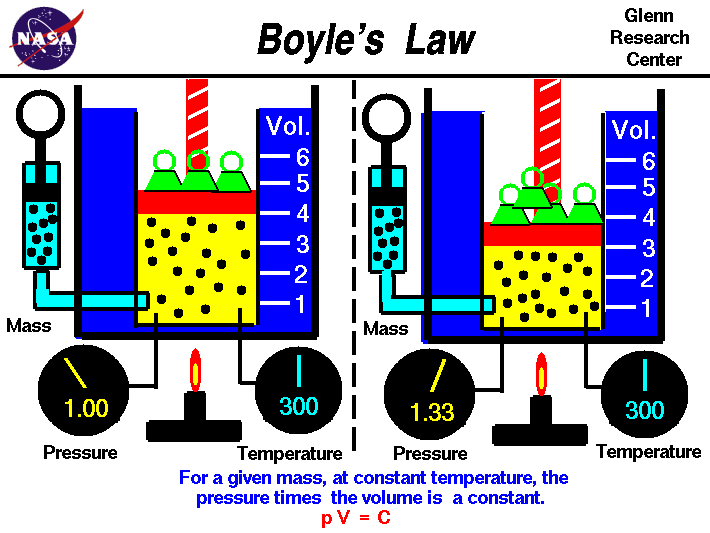
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html

Characteristics of gases
Gases expand spontaneously to fill their container. Gases are also highly compressible and form homogeneous mixtures. To measure the pressure of a gas a barometer is used. A simple barometer consists of a long glass tube, closed at one end and filled with mercury. There are a variety of ways to measure pressure and each have a different unit.
http://www.chem4kids.com/files/matter_gas.html
http://www.mikeblaber.org/oldwine/chm1045/notes/Gases/Intro/Gases01.htm
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch4/properties2.html
https://www.chem.purdue.edu/gchelp/liquids/character.html

http://www.chem4kids.com/files/matter_gas.html
http://www.mikeblaber.org/oldwine/chm1045/notes/Gases/Intro/Gases01.htm
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch4/properties2.html
https://www.chem.purdue.edu/gchelp/liquids/character.html
Energy in all forms
Thermodynamics is the study of energy transformations. In physics, energy is defined as the ability to do work(work=force/distance). Although we are unable to see or directly measure energy, we know that energy has the ability to make things happen. Two common forms of energy in chemistry are kenetic energy, the energy of motion, and potential energy, or stored energy.
Kinetic energy- energy of motion KE=1/2mv^2
Radiant energy- energy from the sun or solar energy
Thermal energy-energy associated with random motion of atoms and molecules
Chemical energy-energy stored within the structural units of chemical substances
Potential energy-energy stored or energy of position


Kinetic energy- energy of motion KE=1/2mv^2
Radiant energy- energy from the sun or solar energy
Thermal energy-energy associated with random motion of atoms and molecules
Chemical energy-energy stored within the structural units of chemical substances
Potential energy-energy stored or energy of position
Cooling and heating
Change of state is a physical change where intermolecular bonds are broken. Melting and boiling points are determined by vapor pressures of the solid and liquid states. At 0 degrees Celsius, ice and liquid water have the same vapor pressure. At 100 degrees Celsius, water vapor and atmospheric pressure are equal.
Super cooling is cooling of liquid below freezing point and it occurs when the particles lack the internal arrangement to solidify at freezing point.
Super heating is heating of a liquid is raised above its boiling point and it occurs when vapor bubbles fail to form and it may suddenly boil violently when vapor bubbles do form.
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/phase.html
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/watice.html
https://www.chem.wisc.edu/deptfiles/genchem/netorial/rottosen/tutorial/modules/intermolecular_forces/02imf/imf2.htm
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/changesOfPhase/changeOfState.html
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/changesOfPhase/changeOfState.html
http://www.shmoop.com/matter-properties/phase-change.html


Super cooling is cooling of liquid below freezing point and it occurs when the particles lack the internal arrangement to solidify at freezing point.
Super heating is heating of a liquid is raised above its boiling point and it occurs when vapor bubbles fail to form and it may suddenly boil violently when vapor bubbles do form.
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/phase.html
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/watice.html
https://www.chem.wisc.edu/deptfiles/genchem/netorial/rottosen/tutorial/modules/intermolecular_forces/02imf/imf2.htm
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/changesOfPhase/changeOfState.html
http://zonalandeducation.com/mstm/physics/mechanics/energy/heatAndTemperature/changesOfPhase/changeOfState.html
http://www.shmoop.com/matter-properties/phase-change.html
The U.S.S.
Once we found out we could use the remaining bio-diesel from other groups, we set sail in finding an idea that would make out putt-putt boat glide past the competition. we ended up making a model out of a soda can because it was light but also durable and was essentially water proof. We placed 4th and didn't get extra credit but we still felt we had a good team effort and planned everything perfectly.

The Making of our Biodiesel
My partner and I started making our bio-diesel and used the clean oil for the construction instead of the used Chick-fil-a oil. our mindset was that since it was cleaner the oil would burn hotter making our future boat run faster. Unfortunately, the solo cups we stored our biodiesel in disintegrated and all that was left was a mess that Mrs. Frankenberg had to clean. All hope is not yet lost. We know our biodiesel would have rocked based on our test burn.
was a mess that Mrs. Frankenberg had to clean. All hope is not yet lost. We know our biodiesel would have rocked based on our test burn.

Biodiesel Project
Today and all the next week we will be in the library computer lab working on our Bio diesel project. Unfortunately my partner wont be able to attend school so i will have to figure out a approach to our video and hope that we can finish in time. overall i'm excited about this project and hope to win the top prize.


http://biodiesel.org/
https://en.wikipedia.org/wiki/Biodiesel
http://www.afdc.energy.gov/fuels/biodiesel.html
https://www.fueleconomy.gov/feg/biodiesel.shtml
http://www.biodiesel.com/
http://www.biodieselmagazine.com/
http://biodiesel.org/
https://en.wikipedia.org/wiki/Biodiesel
http://www.afdc.energy.gov/fuels/biodiesel.html
https://www.fueleconomy.gov/feg/biodiesel.shtml
http://www.biodiesel.com/
http://www.biodieselmagazine.com/
Unit test
Although i studied for the test the whole week and understood the terminology and the mathematical side of it, i did poorly on the test next time i might look on quizlet for more practice. I have put links down below of what i would think are helpful.
https://quizlet.com/
http://www.visionlearning.com/en/library/Chemistry/1/Chemical-Bonding/55
https://en.wikipedia.org/wiki/Covalent_bond
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/Lewis_Theory_of_Bonding
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/lewis.html
https://www.khanacademy.org/science/chemistry/chemical-bonds/copy-of-dot-structures/v/drawing-dot-structures

https://quizlet.com/
http://www.visionlearning.com/en/library/Chemistry/1/Chemical-Bonding/55
https://en.wikipedia.org/wiki/Covalent_bond
http://chemwiki.ucdavis.edu/Core/Theoretical_Chemistry/Chemical_Bonding/Lewis_Theory_of_Bonding
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/lewis.html
https://www.khanacademy.org/science/chemistry/chemical-bonds/copy-of-dot-structures/v/drawing-dot-structures
Lewis Structures
Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Depending on the chemical make up of that bond, the Lewis structure may be different. Some Lewis structures appear flat such as the bond of water today we went to the library to draw Lewis structures on the dry erase tables.







All Shapes and Sizes
A chemical bond is a lasting attraction between atoms that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between atoms with opposite charges, or through the sharing of electrons as in the covalent bonds.
much of what we studied in class was the way the chemical bonds formed and the shapes they created below is a chart of the shapes bonds form
much of what we studied in class was the way the chemical bonds formed and the shapes they created below is a chart of the shapes bonds form
Subscribe to:
Comments (Atom)


