Going in to this lab I was prepared but nervous because it was either a pass or fail when being graded. I was under a lot of pressure ;). Somehow by studying the formula and looking at similar labs on YouTube I made it through the lab with flying colors. I had the smallest bag size and thought that would be the hardest but I'm glad I didn't have to stand at the sink the whole hour measuring the volume of the bag like others with the bigger bags had to do,
Showing posts with label Gas Laws(4). Show all posts
Showing posts with label Gas Laws(4). Show all posts
Tuesday, May 10, 2016
Avagadros law
Avagadros law tells us that for a gas at constant temperature and pressure, the volume is directly proportional to the number of moles of gas present. Equal volumes of gases at the same temperature and pressure have the same number of particles. V1/n1 = V2/n2
http://www.chemteam.info/GasLaw/Gas-Avogadro.html

http://www.chemteam.info/GasLaw/Gas-Avogadro.html

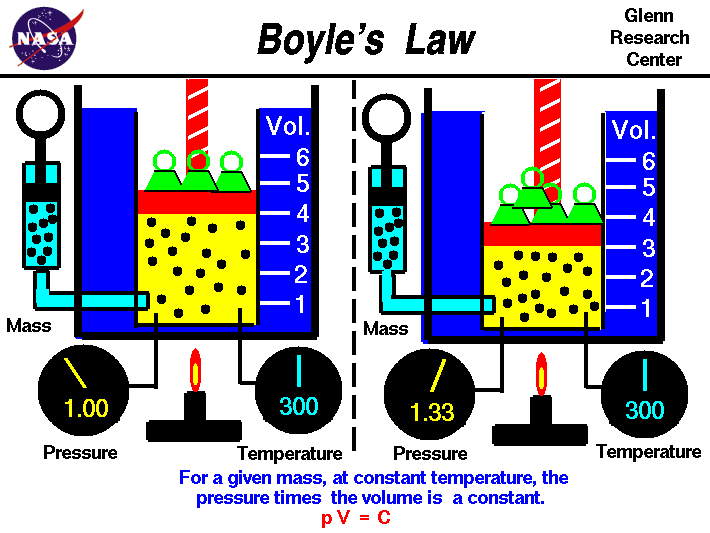
Boyle's Law
Boyle's law tells us that the relationship between pressure and volume is an inverse relationship. It deals with two of the four factors that determine the state of gas: pressure and volume. Temperatures are held constant. This is for ideal gases. As we increase in volume the pressure decreases. But as volume of a container decreases pressure will increase due to increase in collisions.
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html

https://www.grc.nasa.gov/www/k-12/airplane/boyle.html

Characteristics of gases
Gases expand spontaneously to fill their container. Gases are also highly compressible and form homogeneous mixtures. To measure the pressure of a gas a barometer is used. A simple barometer consists of a long glass tube, closed at one end and filled with mercury. There are a variety of ways to measure pressure and each have a different unit.
http://www.chem4kids.com/files/matter_gas.html
http://www.mikeblaber.org/oldwine/chm1045/notes/Gases/Intro/Gases01.htm
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch4/properties2.html
https://www.chem.purdue.edu/gchelp/liquids/character.html

http://www.chem4kids.com/files/matter_gas.html
http://www.mikeblaber.org/oldwine/chm1045/notes/Gases/Intro/Gases01.htm
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch4/properties2.html
https://www.chem.purdue.edu/gchelp/liquids/character.html

Subscribe to:
Posts (Atom)
